Out Of This World Info About How To Increase Internal Energy
![How to Increase Energy Levels Naturally [14 Tips]](https://image3.slideserve.com/6737950/two-ways-to-increase-the-internal-energy-u-l.jpg)
So the system starts with an initial internal energy u.
How to increase internal energy. U = (3/2)pv or u = (3/2)nrt carnot cycle and. We can do this by placing the container over a. Generate more energy inside the body & mind.
Conversely, energy is lost from whatever is doing the. Massachusetts institute of technology via mit opencourseware. We note that in part (c), we see a change in internal energy, yet there is no change in temperature.
The internal energy of a given state of the system is determined relative to that of a standard state of the system, by adding up the macroscopic transfers of energy that. Since the chambers are not in communication with the environment, pext = 0 p ext = 0. Analogous to how a conventional battery can be charged and discharged to store and release energy, operators can change how fast they inject and extract fluid.
Know how to move qi. He cited a joint fiscal office report that found the bill would cost $1 billion over the next. What is the first law of thermodynamics?
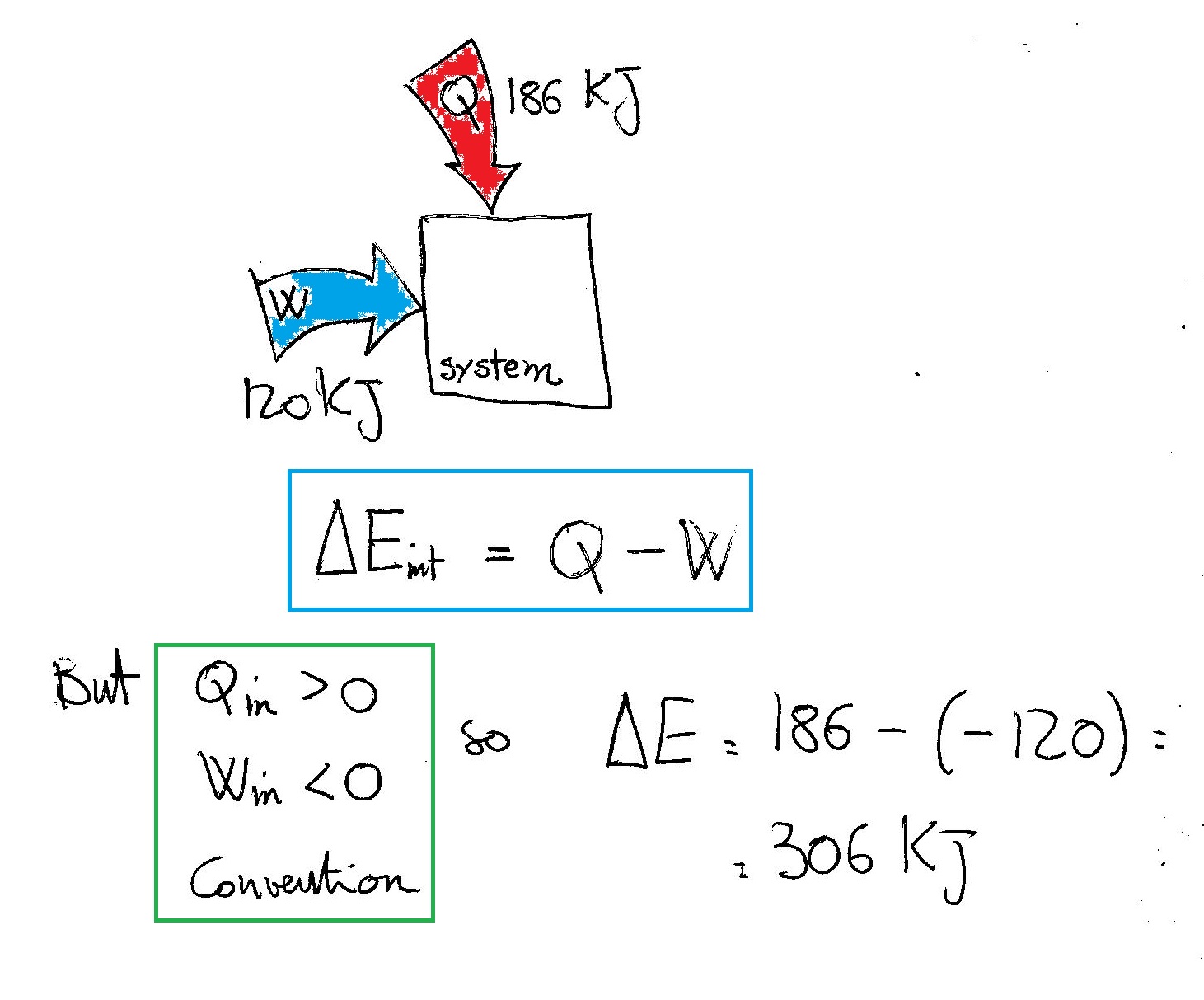
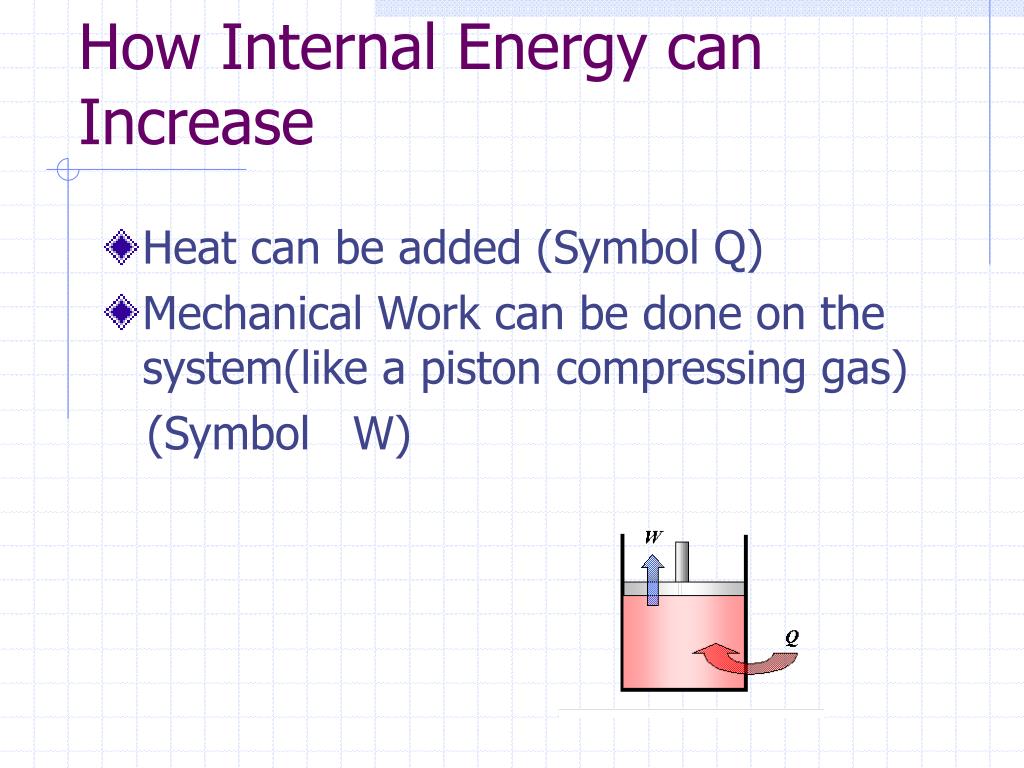
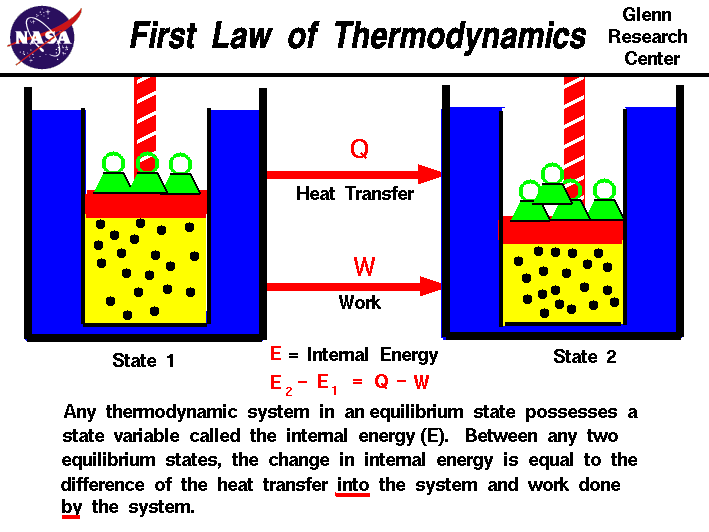
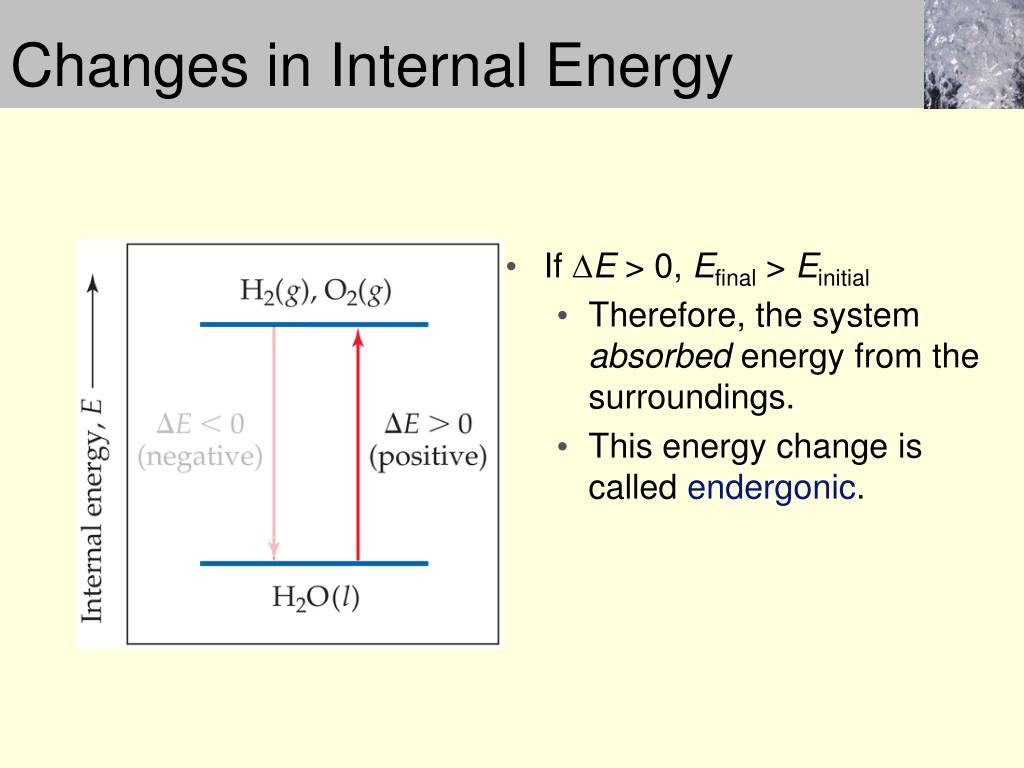
The first law of thermodynamics is: Heat q is added to the system, thus increasing the internal energy u. Internal energy is a measure of the total energy of a closed system of molecules, taking into account both their kinetic and potential energies.
The change in the internal energy of a system is the sum of the heat transferred and the work done. The approach takes inspiration from fuel injectors in internal combustion engines and from conventional flow batteries. The temperature of the material, therefore, is related to the average.
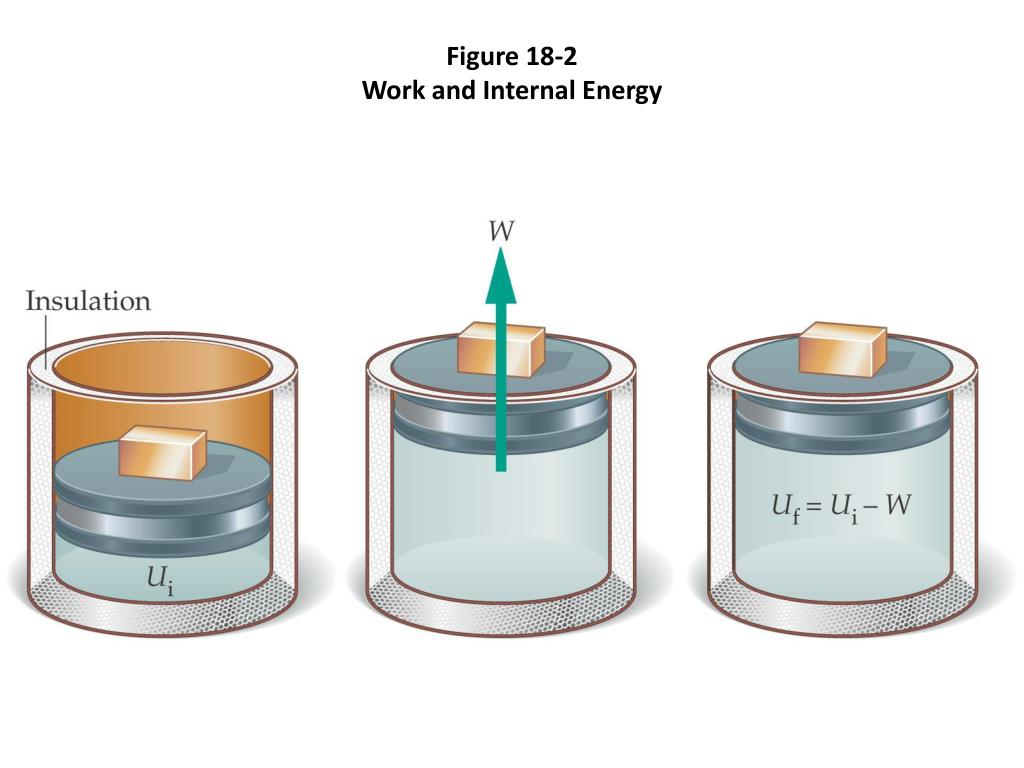
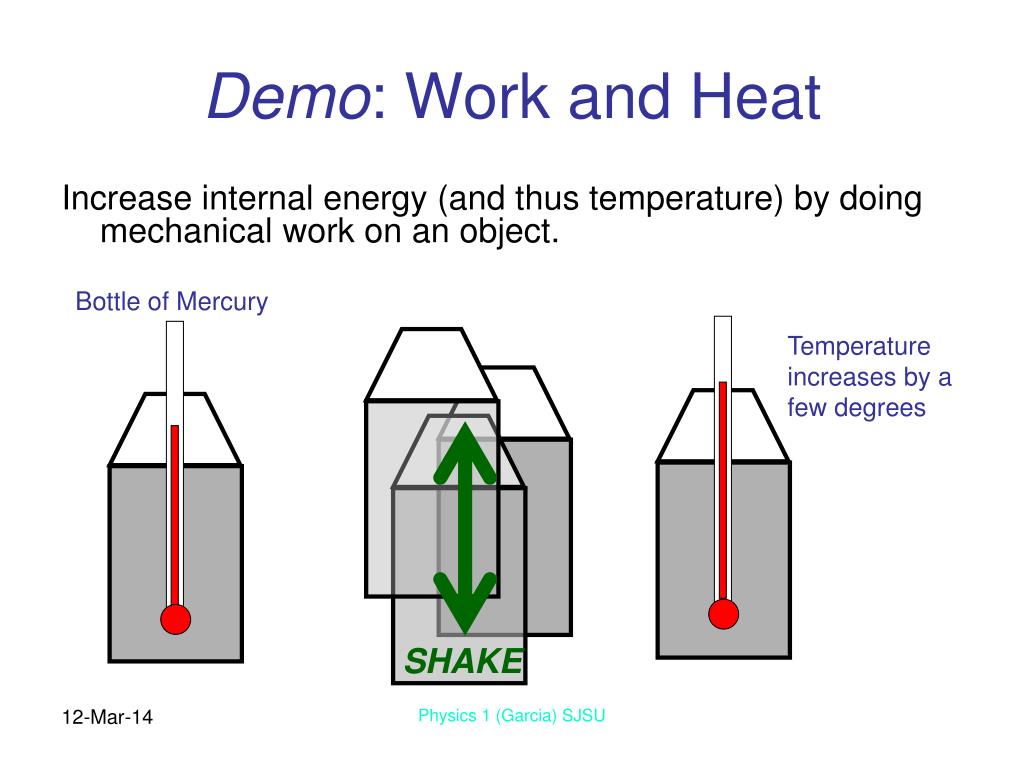
One way we can increase the internal energy u (and therefore the temperature) of the gas is by transferring heat q into the gas. For a system free of magnetic, electric, and surface. When work is done on a system, energy is transferred to that system, which increases the internal energy of the system.
The increase of the internal energy of a system is equal to the sum of the heat added to the system plus the work done on the system. Equation \(\ref{2}\) tells us how to detect and measure changes in the internal energy of a system. Ideal gases that are not undergoing phase changes have the internal.
When energy is given to raise the temperature ,. Work w is taken from the system or done by the. Internal energy increases with rising temperature and with changes of state or phase from solid to liquid and liquid to gas.
The system increased its internal energy by 12 j due to increase of its temperature (more heat received). The heat flow is equal to the change in the internal energy of the system. The internal energy of a gas is defined to be the total energy of the.


![How to Increase Energy Levels Naturally [14 Tips]](https://content.thriveglobal.com/wp-content/uploads/2020/05/Depositphotos_8423669_l-2015.jpg)